Pressurization requirements to prevent cross-contamination.
The dedication of the management and the associates that will work within the room to the Protocols required for the operation and maintenance of the Cleanroom.
Future use of the Cleanroom, including additional equipment, and possible expansion.
Once all of these factors are analyzed, a design can be formulated, using guidelines that have been established, which will lead to a successful “Operational” Cleanroom system. Over design will cost the owner additional upfront cost, and operational cost, and under design will result in production problems.
The more that we can learn about your process, added to our existing knowledge base acquired across a wide spectrum of clients, the more we are able to deliver a design that will meet your requirements today and in the future, while exceeding your expectations.
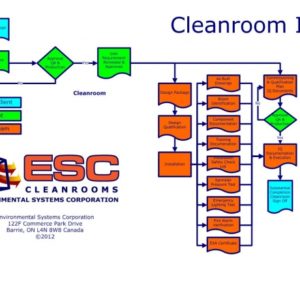
Cleanroom Design
Cleanrooms are designed typically for a specific use, which we will simplistically break down into three Categories:
Based on the primary use, we have simplistic guides to looking at the components required for your Cleanroom
- Electronics & Optics
- Pharmaceutical
- Biological Containment
Review the guides as a starting point for a discussion on what will best suit your present and future needs.
One common error we have encountered is overbuilding at the early stage of a company’s growth, and revenues not being able to fund the facilities built.
Design
Consider a solution that allows flexibility for future growth, and keeps your funding in the bank until the future facility is required. ESC can assist with these decisions required for this plan.
ESC has produced a Cleanroom Design Guide
Download a copy as a starting point for the information to be gathered in the design process.
Contact our office to work through the information on the form, and we will assist you in explaining the requirements.