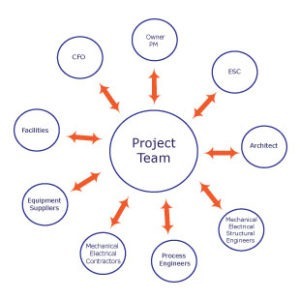
Medical Device Manufacturing Cleanrooms
Medical Device Manufacturing
The manufacture of medical devices is subject to stringent and comprehensive regulations. The type of the medical device determines the cleanliness requirement of the environment.
ISO 13485:2003 is the Medical Devices – Quality Management Systems – Requirements for regulatory purposes most commonly referred to by Medical Device Manufacturers
For most device classes, contamination control is achieved through the use of a Cleanroom facility, which would then refer to ISO 14644.